Contents
What is a Battery?

A stack of batteries
Batteries are energy storage devices. Traditionally, we have used batteries to power small electronic devices such as flashlights. However, you can find batteries in any device that requires a portable power source to function these days.
Because their use cases and purposes can vary, batteries come in all shapes and sizes. For instance, we cannot hope to use AAA batteries to power up a car optimally. Thus, cars require a specially-sized rechargeable battery to function correctly.
How do Batteries Work?
Batteries store electrical energy as chemical energy. When we charge a (rechargeable) battery, it converts the electrical power and holds it as chemical energy. When we use the storm, a chemical reaction occurs, and the battery releases its charge as electricity.
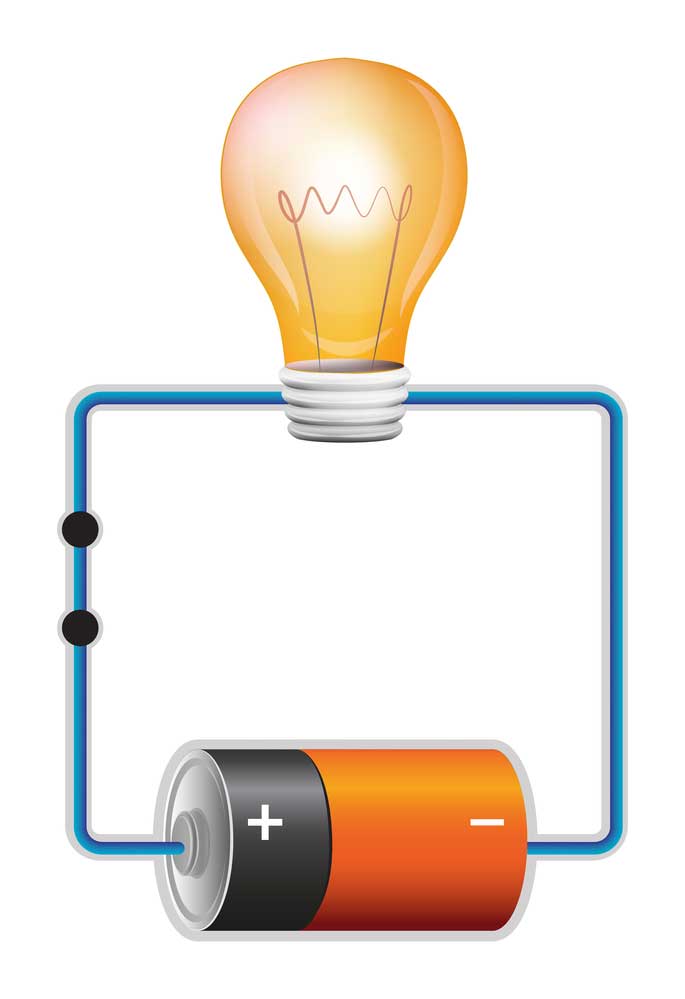
battery and lamp circuit
For instance, the lamp in the above simple circuit will require a sea of electrons to flow through it to function. Accordingly, the battery will provide the pushing force required to propel the electrons through the lamp. We need to connect the light to the positive and negative terminals of the battery to complete the circuit. If there is enough charge in the storm, the lamp will illuminate.
However, it’s important to remember that the battery can only push the electrons through the circuit for a limited amount of time. Essentially, this time limit is dependent on the amount of stored energy (charge) and the demand by the load. In our example, the lamp is our load, and the market refers to the energy required to function. Thus, the bag is any component that requires electricity to work. Concerning PCBs, these components can be diodes, microchips (integrated circuits), etc.
Difference Between a Cell and Battery

A close-up image of various types of batteries
An electric cell is a single-unit component that fundamentally works the same way a battery does – converting chemical energy into electricity. A storm is a group or collection of cells. Nevertheless, the most popular form of an electric cell is the button cell. People also refer to it as a coin battery. This action isn’t necessarily incorrect. A singular cell can be in a group that makes up a battery.
Types of Batteries

Vector images of batteries
Batteries fall into two main categories:
- Primary Batteries: Disposable batteries that we cannot recharge. As such, people sometimes refer to them as single-use or throw-away batteries. The biggest advantage of these batteries is that they can immediately produce current upon assembly. We often use them in portable devices with a low current drain or intermittent uses.
- Secondary Batteries: We often refer to these batteries as rechargeable batteries. Manufacturers typically assemble them with the active materials in the discharged state. Users can then recharge them by applying an electric current. Incidentally, charging reverses the chemical reactions that occur during its use. We perform charging using devices we refer to as chargers and/or rechargers. Recharging and discharging can occur multiple times depending on the battery type (material).
Types of Primary Batteries:
- Zinc-Carbon battery
Zinc-carbon batteries are the lowest-cost household primary battery. They disperse current by using a chemical reaction between zinc-acidic manganese dioxide. However, the most significant disadvantage of zinc-carbon batteries is their low power output. Nevertheless, this also results in a low self-discharge rate, which affords them an exceptional shelf life. Zinc-carbon batteries are most suitable for clocks and remote controllers.
- Alkaline battery

Image of alkaline battery
Pixabay
Non-rechargeable alkaline batteries are the most used household primary battery types. They use an electrochemical reaction between zinc metal and manganese dioxide to hold and disperse charges. Incidentally, their current popularity is derived from their high power-per-use. At least compared to carbon-zinc batteries (and most secondary batteries). Additionally, they tend to have superior shelf lives.
Lithium battery (or lithium metal batteries)
Primary lithium batteries use a metallic lithium material as an anode. Incidentally, they outperform zinc-carbon and alkaline batteries in power-per-use because of their high charge density. Additionally, lithium metal batteries and cells have incredible shelf lives.
They can last well beyond a decade and can work under low temperatures. However, lithium batteries aren’t as popular or readily available as Zinc carbon and alkaline batteries because of their high production costs.
Nevertheless, lithium metal batteries and cells can produce voltages between 1.5V and 3.7V. As such, manufacturers typically package them in smaller form factors.
Larger-sized lithium batteries have a higher risk of instability, but we can still use them in military applications.
Silver-oxide battery
Silver oxide batteries are prevalent in Japan but not so much in the rest of the world. They use silver oxide for their cathodes and zinc for their anodes. While they have a high energy density, they are pretty expensive. We can attribute this to the cathode material (silver) required to manufacture them. Silver is a precious metal. Thus, we mainly use them in coin battery designs where the amount of silver needed is small.
Zinc-air battery
Many people consider zinc-air batteries to be acceptable alternatives to silver-oxide batteries. They use the oxygen from the surrounding air as a cathode while housing the anode inside the electric cell. Because of their high energy density, they are most suitable for medical devices such as hearing aids and pacemakers. Incidentally, we can attribute their long life spans to their makeup and structure.
Mercury battery
Mercury (aka mercury oxide battery/cell or Ruben-Mallory) battery is a non-rechargeable battery that uses an electrochemical reaction between mercuric-oxide and zinc electrodes in an alkaline electrolyte. They have a higher capacity than zinc-carbon batteries and provide a steady and reliable output.
Typically, mercury batteries usually come in the form of coin batteries. They were trendy during WW2. However, it’s nearly impossible to find them these days. However, because of mercury’s toxicity to the environment and human health, many countries have banned their sales.
Types of Secondary Batteries:
- Rechargeable alkaline battery
The rechargeable version of alkaline batteries is the most affordable rechargeable battery. Additionally, they have a long shelf life. They are handy for moderate-power applications. Despite their short life-cycle (compared to other secondary batteries), they are still trendy. Because they do not use toxic materials, users can safely dispose of them in regular landfills – as long as local regulations permit it.
Nickel-Cadmium
Rechargeable nickel-cadmium (Ni-Cd) batteries are well-liked because of their ruggedness and reliability. They have a varied operating temperature range and a long life span. Furthermore, and most importantly, they have high power capabilities. However, they tend to have a low run-time per charge. Another disadvantage is their generally high self-discharge rate (30% per month). But this isn’t a huge problem, considering that they are rechargeable.
Nevertheless, another undesirable feature of Nickel Cadmium is in its makeup. Roughly 15% of most Ni-Cd batteries consist of toxic, carcinogenic cadmium. Thus, consumers and manufacturers may not dispose of them in landfills. We must recycle them carefully.
Nickel-Metal Hydride
Rechargeable nickel-metal hydride (NiMH) batteries are an improved iteration of old nickel-cadmium batteries. They provide the same voltage but offer up to 30% more capacity. Furthermore, they have excellent high-current capabilities and long cycle lives.
Nickel-metal hydride batteries can discharge up to 40% of their charge every month. However, the self-discharge rate of nickel-metal hydride batteries is disappointingly higher than nickel-cadmium batteries. However, nickel-metal hydride batteries are still superior to nickel-cadmium batteries because they do not contain toxic cadmium.
Notwithstanding, they still contain large amounts of nickel oxides and traces of cobalt. These can be carcinogenic to mammalian organisms. Therefore, you should recycle nickel-metal hydride batteries carefully.
Lithium-Ion battery

lithium-ion cell phone batteries
Rechargeable lithium-ion (Li-ion) batteries are the newest type of secondary battery. Generally, they are 30% lighter than nickel-metal hydride. Additionally, they provide at least a third of more power capacity of nickel-cadmium batteries.
Lithium-ion batteries have excellent high-current capabilities. Furthermore, they have long cycle lives. Moreover, they have a self-discharge rate of 20% per month. Thus, we can surmise that lithium-ion batteries are superior to cadmium-based batteries in nearly every way. However, they are not as robust. For instance, lithium-ion batteries are susceptible to overheating, which may cause fires.
Additionally, while they’re free from toxic cadmium, most lithium-ion batteries still contain traces of cobalt oxides and nickel oxides. Again, these chemicals are carcinogenic and destructive to human health. Thus, you must dispose of and recycle lithium-ion batteries carefully.
Lead-acid battery

Lead-acid batteries are commonly part of a motor vehicle’s electrical system
Lead-acid batteries are the most sought-after rechargeable battery types in the world. This is mainly due to their reliability and the financial feasibility of their production. Nevertheless, they aren’t very portable because they tend to be bulky, heavy, and oversized. Furthermore, lead is a highly toxic carcinogenic chemical compound.
Therefore, we mustn’t dispose of lead-acid batteries in regular landfills and waste sites. Manufacturers recycle and reuse old dead and damaged lead-acid batteries to produce new lead-acid batteries.
Why Aren’t All Battery Types Rechargeable?
Battery charging vector image
When we recharge a secondary battery, not only do we force electrons back into the storm, but we revert the chemical materials within the battery to their initial charged state. When manufacturers create primary batteries, they assemble the materials in a charged state. Using the charge in a primary battery permanently reconfigures the materials within it. After the reconfiguration process, it cannot hold another order.
On the other hand, secondary batteries activate a chemical reaction to output charge and return to their original status through recharging. Of course, they have to use certain materials and chemicals compatible with these changes.
Battery Applications

Man checking a car battery
Some of the most common applications for batteries include:
- Alarms
- Communications circuits
- Clocks
- Remote controls
- Pacemakers
- Hearing aids
- Wireless headsets
- Motor vehicles
- Wireless computer peripherals (keyboards and mice)
- Handheld gaming devices (Game Boys)
- Smartwatches
How to Choose a Battery?

A woman holding a stack of different types of batteries
Now that you’re familiar with all the available battery types, you need to decide which one is best for your project. When people select batteries, they tend to balance cost and performance. However, there are other specifications you should consider. These include:
- Battery type: Primary (non-rechargeable) or secondary (rechargeable)
- Capacity: The amount of energy/electricity a load can discharge from a battery. We usually denote this using amp-hr. For instance, a 500mAh battery can offload one milliamp per hour.
- Shelf life: The amount of time a battery can keep its charge while sitting unused.
- Battery operating temperature: Depicts how hot a battery becomes while you’re using or recharging it. Consequently, this specification will help you determine if you need a heat sink or cooler for your battery.
- Recharge rate: Describes how quickly a battery a rechargeable battery can recharge
- Battery life: Describes the number of times we can recharge and discharge a secondary battery. Alternatively, we may use it to denote how long we can use a primary battery.
Conclusion
There are no single-battery solutions on the market for all portable battery-operated devices. Because electronic devices come in all shapes, sizes, and ruggedness, they require a power source that is most suitable for their space and load requirements. Thus, a variety of batteries exist with their advantages and disadvantages. The above guide highlighted this by exhibiting all the currently available types of batteries. Remember to dispose of and recycle used batteries with care. Nevertheless, we hope that you have found this guide to be helpful. As always, thank you for reading.