What do you do when you want to test pH levels? Simple! You get a pH meter.
But, there are various types of pH meters—making it tricky to choose the best one for your testing needs.
Fortunately, the pH meters made their debut in 1936.
Arnold Beckman was the first to create a fully functional model tagged “Madel G.”
Thankfully, pH meters are more widespread among lab scientists and chemists.
But what is a pH meter?
And how can you use it to perform tests?
In this article, we’ll answer these questions and provide more details on the types of pH meters.
Contents
What is a pH Meter
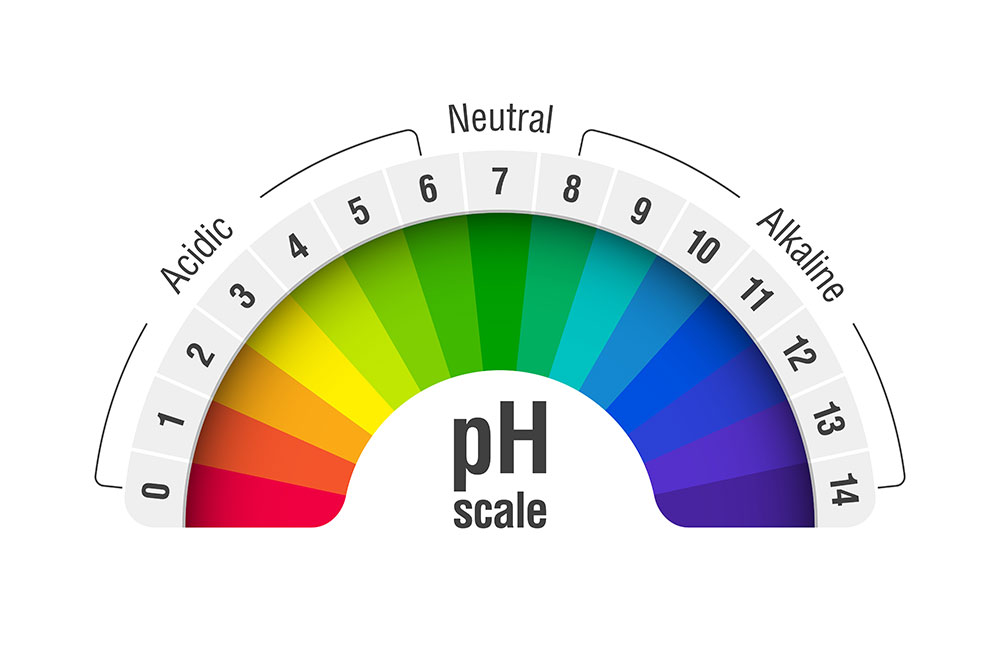
pH scale
It’s easy to mistake pH probes for pH meters, so we must differentiate them to understand their concepts. pH meters are instruments used to measure a liquid’s or solution’s overall alkalinity or acidity.
Typically, this device measures acidity and alkalinity on a 0 to 14.
So, seven on this scale is neutral, while anything below this value is acidic.
In contrast, solutions with values above seven are considered alkaline.
On the other hand, pH probes account for the complete measuring component connected to the meter.
Usually, probes comprise an electrode, a preamplifier with a temperature sensor, and a housing unit.
In addition, the pH element is a thin glass membrane through which hydrogen ions can permeate.
Types of pH Meters
You can access three primary types of pH meters, each offering unique benefits.
Let’s take a closer look at these types.
pH Electrodes

pH electrode
Also called pen testers, pH electrodes are the market’s most common and affordable meters.
In addition to being widespread and cheap, these pen testers are incredibly easy to use.
In truth, its design places the electrode in a glass container shaped like a pen.
Plus, the tip of this tool is particularly sensitive to hydrogen ions. pH electrodes are small enough to transport and use anywhere.
However, its uses are limited compared to other pH meters.
Nevertheless, pH electrodes are the go-to for construction, pool maintenance, food manufacturing, and hydroponics testing.
Also, there are two major pH electrodes: double and single junctions.
Double Junction
Double junction pH electrodes have salt bridges to prevent unwanted reactions between your sample and the electrode-filled solution.
Such reactions may damage the electrode junction without the proper measures in place.
Therefore, you can use these pH electrodes to test samples containing heavy metals, proteins, or sulfides.
Single Junction
In contrast, single-junction pH electrodes work for sample tests without the potential to block or damage the junction.
Handheld pH Meters

A person using a handheld pH meter
Handheld pH meters offer more heavy-duty testing than pH electrodes.
Usually, they comprise a handheld device for calibration & display and an interchangeable electrode probe component.
Environmental officers often use these meters for fieldwork in water treatment, aquaculture, and agriculture industries.
In addition, most modern variants provide wireless technology and Bluetooth features for easier data transmission.
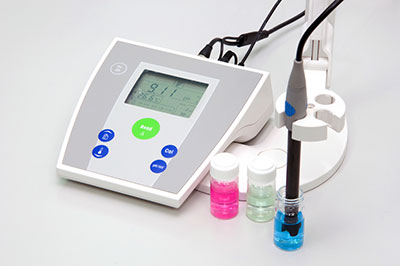
Benchtop pH Meters

Benchtop pH meter
These are the pH meters usually prominent in professional industries and laboratories.
Benchtop meters offer the biggest meter size and are often wall or desk-mounted.
Benchtop pH meters offer the highest accuracy when testing pH levels.
Therefore, you’ll find them in food processing facilities, water testing, quality assurance, and environmental testing.
How to Calibrate and Use pH Meters
Here are the steps to ensure accurate readings, regardless of the pH meter type.
- Switch on the meter and allow it to run for a few minutes.
- Next, rinse the electrode with distilled water to remove unwanted particles. While at it, remember to avoid touching or letting the electrode contact other solutions–so it won’t get contaminated.
- Prepare your buffers for meter calibration. You can pour each into separate containers, allowing them to reach similar temperatures.
So, one buffer should be neutral (seven on the scale), while the other with a pH close to what you expect from your sample.
Further, low pH buffers work for acidic solutions, while high pH buffers can test alkaline bases.
- Start calibrating by testing your pH meter in the neutral buffer.
- Then, adjust the pH meter value to match the neutral buffer’s readings.
- Clean your electrodes again in distilled water.
- Repeat the test for the buffer matching your sample’s pH level.
- Next, adjust your pH meter’s value according to the sample pH buffer readings.
- Rinse the electrode again in distilled water.
- Start your sample test and measure the pH level.
- Finally, clean the electrode after use.
Final Words
Multiple pH meters
You’ll need a pH tester if you’re in the testing field.
These devices can provide accurate readings of the acidity and alkalinity of any liquid or solution.
In addition, you can use the three types of pH testers for different applications.
For instance, pH electrodes are perfect for testing on the go, while handheld devices are the go-to for field testing.
Finally, the benchtop pH meters offer the peak of heavy-duty pH testing with incredibly accurate results.
Do you have any questions on this topic? Be sure to contact us, and we’ll be sure to assist.